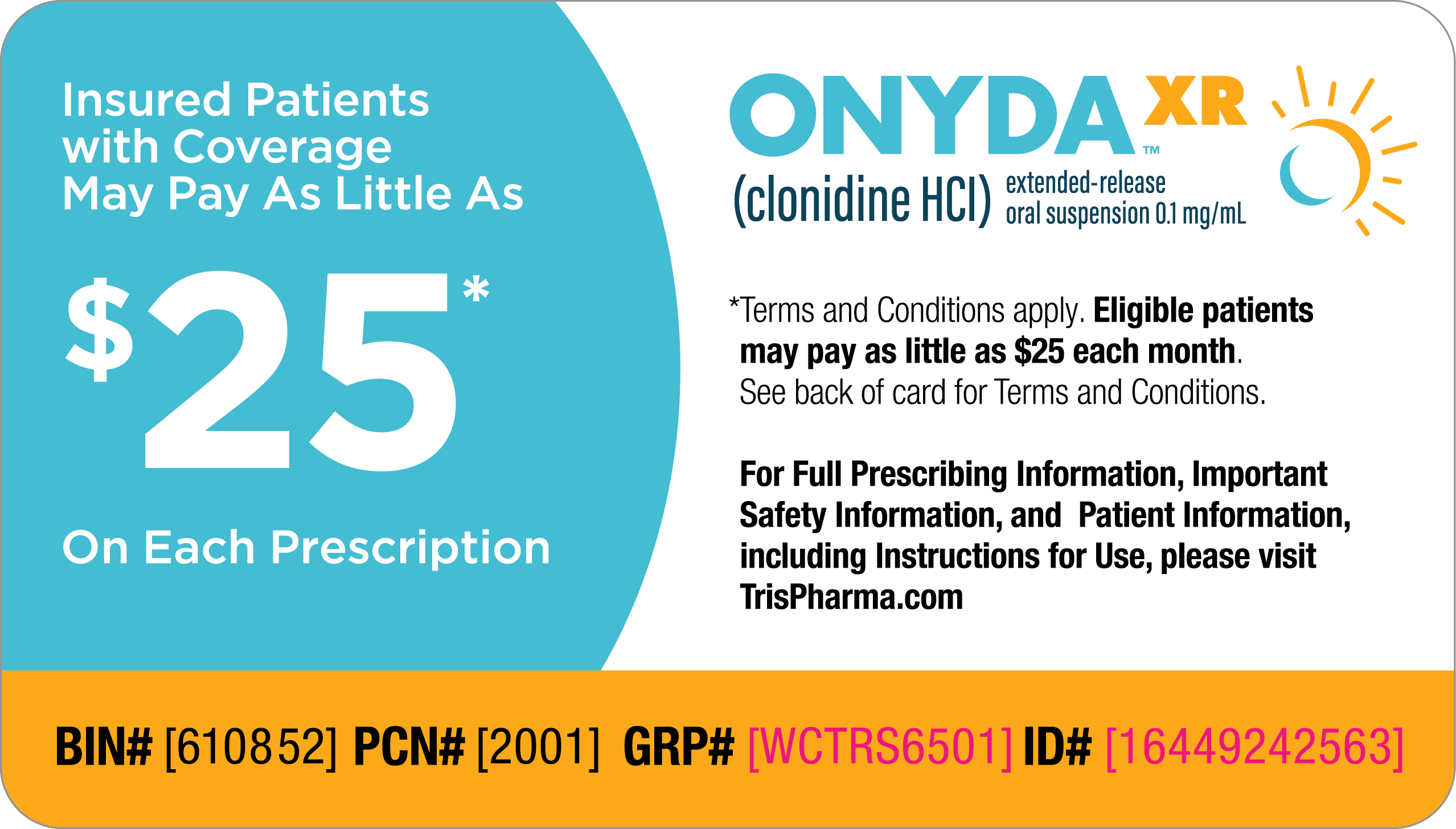
ONYDA™ XR (clonidine HCI) is a prescription medicine used for the treatment of attention deficit hyperactivity disorder (ADHD) alone or with certain other ADHD medicines in children 6 years of age and older. It is not known if ONYDA XR is safe and effective in children under 6 years of age.